The pharmaceutical logistics sector in the Middle East is rapidly evolving — and 2026 promises even greater regulatory complexity, operational pressure, and compliance risk. For supply chain professionals, understanding these risks is not optional; it’s mission critical.
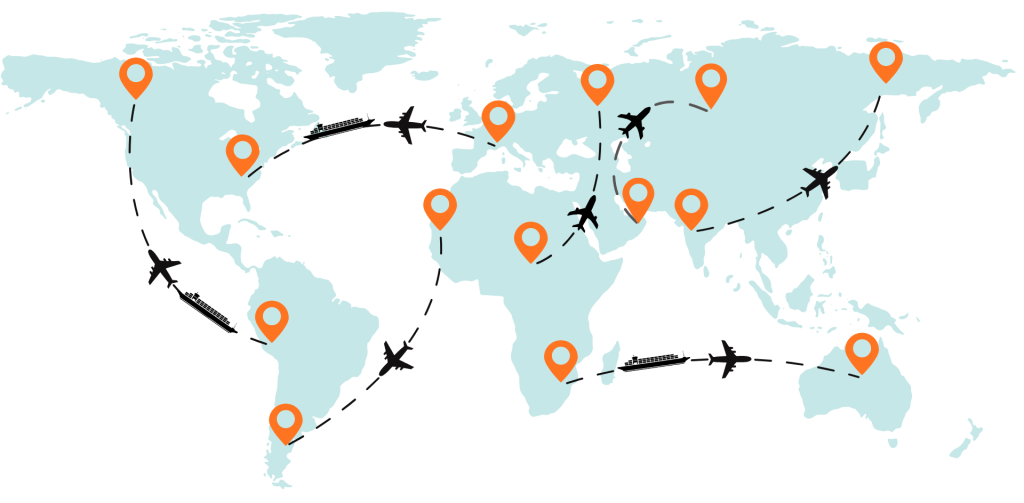
In markets like the UAE, Saudi Arabia, Qatar and the wider GCC, pharmaceutical imports and distribution must align with stringent regulatory frameworks while ensuring temperature‑sensitive shipping remains compliant, efficient and secure. This guide breaks down the top compliance risks, why they matter, and how to mitigate them — including through strategic partners like Arib Shipping.
1. Rapid Regulatory Change Requires Constant Vigilance
Regulatory landscapes in the UAE & Middle East update frequently, and deviations can lead to delays, fines, shipment holds or outright bans.
-
Local health authorities, like the UAE Ministry of Health and Prevention (MOHAP), enforce strict Good Distribution Practices (GDP) and documentation rules for pharmaceutical logistics.
-
Each market may have unique customs protocols, Arabic labeling requirements, and local registration rules — especially in Saudi Arabia, Egypt and GCC states.
-
Frequent audit cycles mean logistics teams must maintain perfect records every time. Errors in documentation, batch numbers, temperature logs or missing certificates can trigger inspections and holds.
Compliance Tip: Integrate dynamic SOPs and automated checks so regulatory shifts are captured and enforced in real time.
2. Documentation Errors: The #1 Cause of Customs Delays
Documentation is the backbone of pharma compliance — and also its biggest vulnerability.
-
Incomplete or inaccurate documentation is a leading contributor to customs delays.
-
Standard paperwork required for imports includes commercial invoices with HS codes, packing lists, AWBs/Bills of Lading, Certificates of Analysis (CoA), import/export permits, and chain‑of‑custody data.
-
Language and translation issues also create risks; technical terms must be rendered accurately for customs clearance.
Impact: A single missing document can hold a shipment at port for days, causing temperature excursions — and potential loss of product integrity.
Mitigation: Adopt centralized digital document management systems with automated validation checks.
3. Temperature‑Sensitive Shipping: Cold Chain Integrity Under Scrutiny
Pharmaceutical logistics isn’t just about moving boxes — it’s about moving life‑critical, temperature‑sensitive products across regions with high heat.
-
Vaccines, biologics and many modern therapies demand a strict cold chain (e.g., 2°C–8°C or lower)
-
The Middle East’s hot climate compounds cold chain challenges, especially during ground transport or customs holds.
-
Temperature excursions risk product efficacy, regulatory breach and patient safety — costing companies millions in wasted medication and compliance penalties.
Best Practices:
-
Active temperature monitoring using IoT sensors reporting real‑time data.
-
Redundant cooling systems and validated packaging solutions.
-
Qualified cold chain carriers trained in Good Distribution Practices.
4. Customs Bottlenecks & Cross‑Border Complexity
Customs compliance in the Middle East is not uniform.
-
Different markets impose distinct requirements on permits, certificates of origin, and local registration entities.
-
Even under harmonization efforts, frontline customs officers may interpret rules differently, creating unpredictable bottlenecks.
-
High‑value pharma cargo attracts increased scrutiny, which slows processing unless all protocols are perfectly aligned.
What Works: Partnerships with AEO‑certified logistics providers — such as Arib Shipping — ensure procedures meet global security standards, reduce port dwell time and minimize surprises at checkpoints.
5. Security, Counterfeiting & Product Integrity Risk
Pharmaceutical products aren’t just sensitive — they’re valuable. This makes them targets for theft, tampering and counterfeiting.
-
Counterfeit medicines are a real risk in global supply chains — creating legal liabilities and public health threats.
-
Secure transport, tamper‑proof packaging and robust handling protocols are essential elements of compliance and product safety.
Security Experts Recommend:
-
Tamper‑evident seals
-
Blockchain traceability for high‑value batches
-
Zero‑trust supply chain frameworks with continuous verification
6. Audit Preparedness & Internal Compliance Systems
Compliance isn’t a “once‑off” event — it’s continuous.
-
Companies must be ready for sudden regulatory audits requiring full traceability of temperature logs, shipment movements, compliance documentation, handling procedures and personnel records.
-
Internal gaps often stem from decentralized systems, lack of staff training, and manual recordkeeping — all ripe for error.
Pro Tip: Periodic internal audits, centralized data systems, and AI‑assisted tools can significantly reduce risk exposure.
7. Emerging Technologies as Compliance Enablers
In 2026, logistics compliance is not just about avoiding risks — it’s about using technology to predict and prevent them.
-
IoT sensors with cloud dashboards provide real‑time temperature and location data.
-
Blockchain systems create tamper‑proof records of every cold chain step, improving visibility and audit defensibility.
-
AI and machine learning are increasingly used for predictive risk assessment and documentation accuracy.
8. Why Logistics Partners Matter More Than Ever
A strategic logistics partner does more than move cargo — they act as compliance guardians.
An AEO‑certified logistics provider like Arib Shipping offers:
✔ Faster customs clearance with authorized processing lanes
✔ Reduced compliance and documentation risk
✔ Temperature‑controlled cold chain expertise
✔ Transparent, real‑time cargo visibility
✔ Secure movement across the Middle East and global markets
Conclusion: Compliance Equals Competitive Advantage
In 2026, the Middle East’s pharmaceutical logistics landscape demands agility, precision and compliance excellence. Temperature‑sensitive products cannot afford delays or missteps — and regulatory violations translate into real financial, operational and reputational losses.
By understanding risks — from documentation errors to customs bottlenecks, cold chain integrity to evolving regulations — pharma companies and logistics partners can build resilient, compliant, and future‑ready supply chains.
Pharma logistics in the Middle East is not just about transportation — it’s about trust, safety and delivering life‑critical care under the highest standards of compliance.














Leave a Reply