Clinical Trial Logistics
Precision That Powers
Breakthroughs
At Arib Shipping, we understand that every clinical shipment carries more than samples it carries the potential to save lives.
Our Clinical Trial Logistics division is built exclusively to support the research sectors with end-to-end, compliant, and temperature-controlled logistics solutions across the UAE, Middle East, and Europe.
Reliable Logistics for
Critical Research
Clinical research requires absolute accuracy, predictable timelines, and validated cold-chain integrity. We manage each shipment as a time-sensitive, high-value mission, backed by our trained healthcare logistics team, GDP-compliant infrastructure, and real-time visibility systems that track every movement — from collection to delivery.
Our operations ensure that biological samples, investigational drugs, and clinical materials are under the right conditions, documentation, and oversight — every single time.
What We Deliver?
⤷ Validated Cold-Chain
Precision temperature control for sensitive pharma & clinical shipments.
⤷ End-to-End Coordination
Seamless movement from lab to hospital, globally connected.
⤷ Regulatory & GDP
Fully aligned with global pharma logistics standards.
⤷ Time-Critical & Controlled
Rapid, reliable transport with real-time delivery visibility.
⤷ Data-Driven & Monitoring
Smart tracking ensures transparency across every shipment.
Why Leading Researchers Choose Arib Shipping?

Clinical & Pharma Logistics Expertise
Decades of proven excellence in healthcare logistics solutions.
Trained Clinical Logistics Specialists
Expert teams ensuring precision handling at every stage.

Unmatched Delivery Reliability
On-time, every time — even for time-critical shipments.

Accredited Global Partnerships
Trusted storage and distribution through certified facilities.
Seamless International Operations
Effortless customs and compliance for cross-border movement.

24×7 Control Tower Support
Round-the-clock visibility and proactive shipment management.
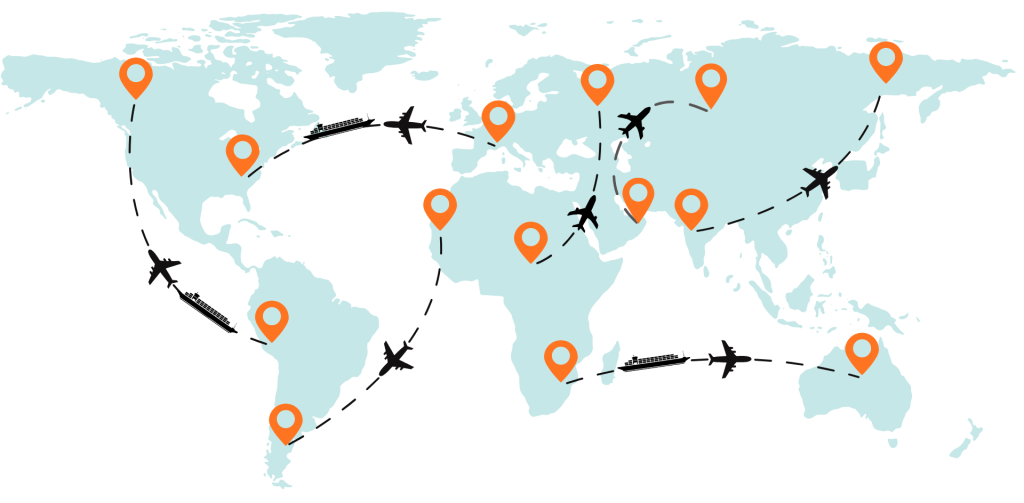
Regions We Cover
We operate across the UAE, GCC, and Europe, enabling cross-border clinical trial logistics through our certified partner network.
Whether you’re shipping investigational medicines, lab samples, or trial kits, Arib Shipping ensures unbroken cold-chain integrity and data-backed compliance at every stage.
Commitment to Compliance & Excellence
Our infrastructure, protocols, and trained specialists work together to maintain the highest global standards. Every process — from packaging to delivery — is auditable, traceable, and fully validated under GDP and ISO frameworks.
This is why leading pharmaceutical companies, CROs, and research facilities trust us to manage their most sensitive consignments.

Partner With Us
Your research deserves a logistics partner that understands its significance.
At Arib Shipping, we ensure your clinical trial materials arrive on time, intact, and in full compliance – every single time.
Contact our Clinical Logistics Team to discuss your next shipment or set up a dedicated trial movement plan.
Our Clients
Trusted by Global
Healthcare Leaders.